As we have seen, there is no possibility that gastric sleeve surgery could be reversed. It removes the majority of the patient’s stomach forever. A gastric bypass is not reversible either.
Another choice would be to install a silicone band that closes off most of the stomach so the tiny remaining pouch can only hold a little bit of food. If a person wanted to leave open the “undo it” option, laparoscopic adjustable gastric band surgery was the way to go — until recently.
Less than a year ago the Food and Drug Administration approved a device designed for short-term use, that is meant to stay in place for only six months. Like the various types of bariatric surgery, it makes most of the stomach inaccessible. However, it will be removed, leaving the patient with entrails intact but hopefully cushioned in much less stomach fat.
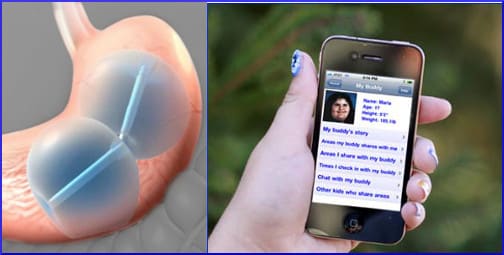
Its official name is the ReShape Dual Balloon, and it works by the intuitive and almost crudely simple method of occupying most of the stomach’s real estate, so that the person feels full.
The study that won government approval for the invention had 326 people in it, all obese, and all with at least one comorbidity. Poundage alone is not enough of a qualifier. In fact, the balloon is supposed to be prescribed for people with a BMI of 30 to 40, who have an obesity-related health problem like diabetes or hypertension. As always with this type of product, the FDA only wants it sold to people who are unable to lose weight through diet and exercise.
The study participants ranged in age from 22 to 60. Half of them were in the balloonless control group, serving as a baseline for comparison. The no-balloon faction lost about 3.3% of their body weight, while the balloon people lost more like 6.8% of their starting weight.
The people equipped for six months with a ReShape Dual Balloon lost an average of 14 pounds. Furthermore, they were followed up six months after removal of the device, and still weighed an average of 10 pounds less than they had the year before.
What happens?
The balloons are definitely contraindicated for anyone with delayed gastric emptying, or irritable bowel syndrome, or any kind of bowel disease, really. No one can have this who is “unable or unwilling to take prescribed proton pump inhibitor medication for the duration of the device implant.”
Pregnant women are excluded, as are daily users of aspirin, and those with active H. pylori infections. Actually, the list of disqualifying conditions is quite long. Saddest of all, the FDA says “adults only,” although that may change.
With the patient mildly sedated, the two connected balloons are inserted into the stomach through the mouth, and then filled with a sterile solution of blue dye, and sealed shut. The dye allows the patient to see if a balloon breaks. After the procedure, it is necessary to keep a close eye on what comes out. Commercial toilet sanitizer that turns the water blue will not be helpful at this stage.
While a half-hour endoscopic outpatient procedure is preferable, by many degrees of magnitude, to surgery, the ballon technology is not without risk. News9.com reported:
These included some discomfort linked to the sedation needed during the insertion procedure, and “rare cases” of severe allergic reaction, heart attack, esophageal tear, infection and breathing difficulties, the agency said. “Once the device is placed in the stomach, patients may experience vomiting, nausea, abdominal pain, gastric ulcers and feelings of indigestion,” the FDA added.
As always, the patients are expected to pursue a medically supervised reasonable diet and workout schedule, during the six months when the device is implanted, and also for the remainder of their lives. This identifies them as yet another group of people who can benefit substantially from everything offered by Dr. Pretlow’s W8Loss2Go smartphone app.
Your responses and feedback are welcome!
Source: “FDA approves ‘belly balloon’ device for weight loss,” News9.com, 07/29/15
Source: “ReShape Integrated Dual Balloon System — P140012,” FDA.gov, 2015
Image by FDA.

 FAQs and Media Requests:
FAQs and Media Requests: 











